Evaluation of corrosion resistance of anodized and passivated aluminum
Question
Anodizing is a surface treatment commonly applied to aluminum, titanium or other metals that can be passivated to improve their surface properties (corrosion resistance, hardness, coloring, etc.). By choosing the right electrolyte, the anodizing process can lead to the formation of a porous columnar structure that can, for example, be impregnated with a colorant. Our analytical techniques can be used to characterize this structure.
For certain applications, it is necessary to seal these pores, particularly when the material's conditions of use call for good chemical and/or corrosion resistance.
In this case study, we show how to quickly assess the quality of a sealed porous anodizing layer?
Expertise
Materia Nova has long-standing expertise in the anodizing of aluminum, titanium and niobium, as well as in pilot-scale application equipment and evaluation methods. In addition to conventional characterization techniques (salt spray, hardness, etc.) Materia Nova has set up a platform for characterizing anodic layers, combining electrochemical impedance measurements (EIS) and microscopic examinations (SEM).
In this case study, 1050 aluminum was anodized in a sulfuric medium. Impedance measurements are used to assess the corrosion protection offered by the anodic layers. The technique is highly sensitive to the quality of the porous layer, and enables relevant information to be obtained in a very short time (a few hours of contact).
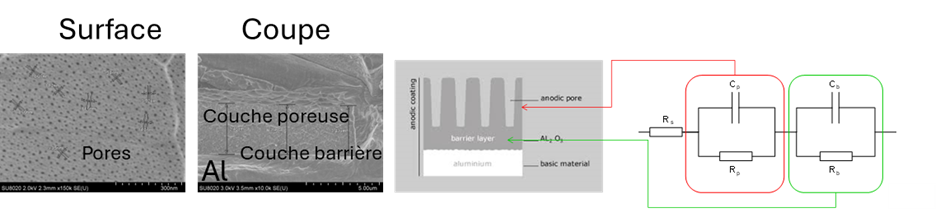
In the case of sulfuric-anodized aluminum, the system consists of a barrier layer and a porous layer. If anodizing is followed by a sealing step, the two characteristic contributions of the barrier layer (in the low-frequency range) and the porous layer (in the high-frequency range) are generally distinguished. This system can be represented by an equivalent electrical diagram in which Cp and Rp represent the capacitance and resistance of the porous layer, and Cb and Rb the capacitance and resistance of the barrier layer.
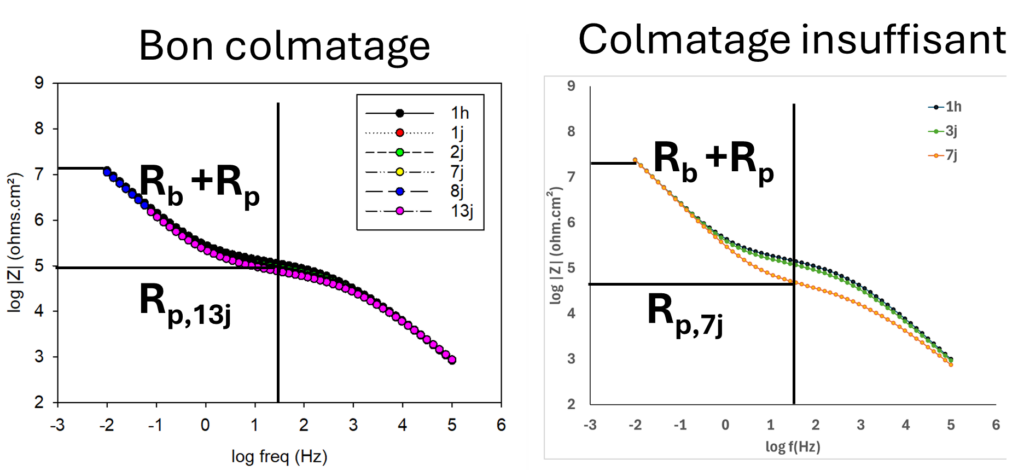
The low-frequency impedance modulus provides information on the corrosion resistance of anodized and sealed aluminum. The higher the value, the greater the corrosion resistance. The impedance spectra shown here illustrate the response of anodized and properly or inadequately sealed aluminum. In the first case, the resistive contribution of the porous layer (intermediate frequencies) remains at 105 Ohms.cm² for up to 13 days of contact with the aggressive solution. If sealing is insufficient, the resistance of the porous layer decreases over time, as shown here. By using suitable software, it is possible to analyze in greater detail the evolution of the parameters characterizing the barrier and porous layers. Determination of the barrier layer's capacitance enables us to estimate its thickness, by virtue of the relationship that links the capacitance of a capacitor to the thickness and dielectric constant of the material (known for alumina). In this case, for example, the barrier layer thickness is 17 nm.
Solution
The EIS technique has enabled us to demonstrate, in a relatively short time, the quality of the porous anodizing layer on 1050 aluminum, and to determine the thickness of the barrier layer. Complementary SEM examinations enable surface observation of the samples and analysis of their porosity (average pore size, density) using suitable image analysis techniques. A cryofracture technique can be used to rapidly obtain the thickness, as well as a cross-sectional morphological examination.